1 Method Of Cracking In Chemistry
For best results in paraffin cracking focus. Cracking is defined as the process of breaking down long chain hydrocarbons into simpler forms or light weight hydrocarbons.

Hydrocarbon Cracking And Why It Is Done The Chemistry Journey The Fuse School Youtube High School Chemistry Organic Chemistry Chemistry
There is a greater demand for smaller hydrocarbons than larger ones.
1 method of cracking in chemistry. Long chain hydrocarbons can be broken into. Catalytic cracking and steam cracking As the names suggest one method uses a catalyst and the other uses steam. Cracking in petroleum refining the process by which heavy hydrocarbonmolecules are broken up into lighter molecules by means of heat and usually pressure and sometimes.
Various methods can be used for cracking eg catalytic cracking and steam cracking. Thermal method of cracking was the first method of hydrocarbon cracking to be developed. The process is as.
Types of Cracking 1. Cracking allows large hydrocarbon molecules to be broken down into smaller more useful hydrocarbon molecules. This will burn hot enough to initiate the reaction without melting the pipette.
Crude oil often contains too many large hydrocarbon molecules and not enough small hydrocarbon molecules to meet demand. Decane octane ethene. Add to My Bitesize Add to My Bitesize.
Catalytic cracking uses a temperature of approximately 550C and a catalyst. C 10 H 22 C 8 H 18 C 2 H 4. There are two methods used to crack alkanes.
Cracking helps match supply of fractions with demand. Cracking is the process whereby long and medium chain hydrocarbons are heated and break apart giving short chain alkanes alkenes and hydrogen. Chemistry Single Science Organic chemistry.
Fractions containing large hydrocarbon molecules. This is where cracking comes in. Thermal Cracking The thermal method of cracking uses pressure and heat to break down large hydrocarbon molecules into.
Cracking in chemistry is of two types. Cracking Cracking is a reaction in which larger saturated hydrocarbon molecules are broken down into smaller more useful hydrocarbon molecules some of which are. Cracking its Products There is a much greater demand for shorter hydrocarbon than there is for the longer hydrocarbons.
Use an ethanol spirit burner for heating. Cracking Chemistry is a selection of fun chemistry practicals developed by National Science Engineering Week curated by the Royal Society of Chemistry.
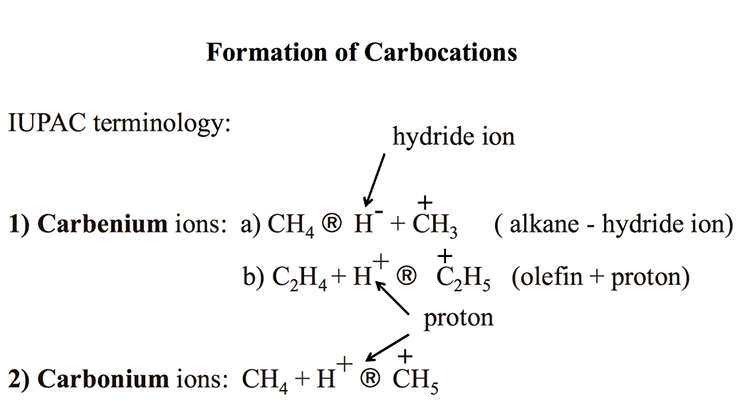
Chemistry Of Catalytic Cracking Fsc 432 Petroleum Refining

Alkenes Cie Igcse Chemistry Revision Notes
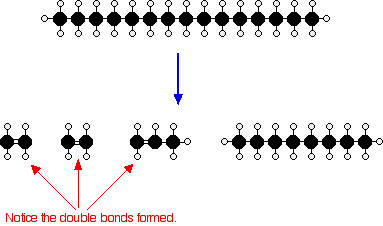
Cracking Alkanes Chemistry Libretexts

Roll To Recap Gcse 9 1 Chemistry Revision For Topic 10 Electrolysis Teaching Resources Chemistry Revision Gcse Chemistry Chemistry
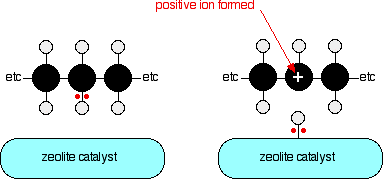
Cracking Alkanes Chemistry Libretexts
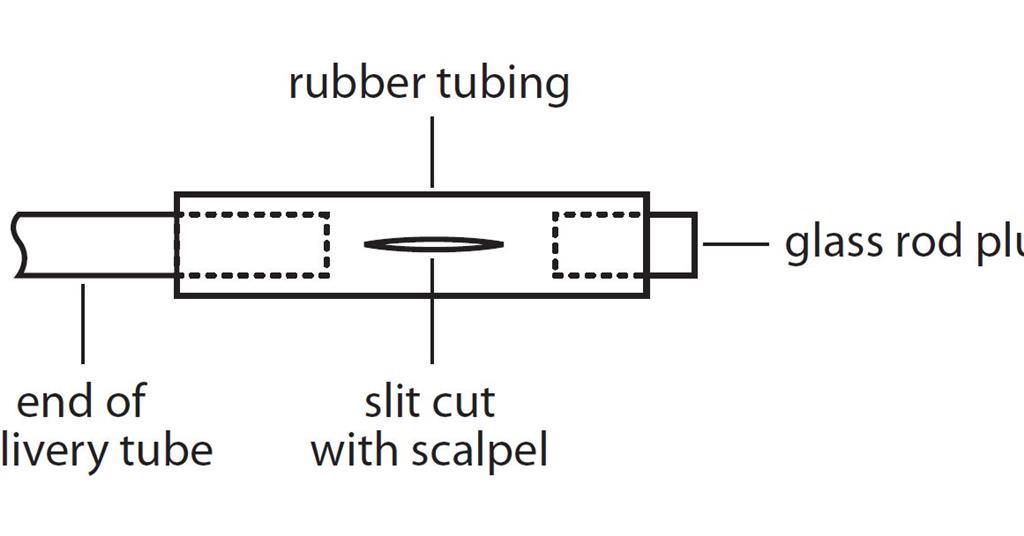
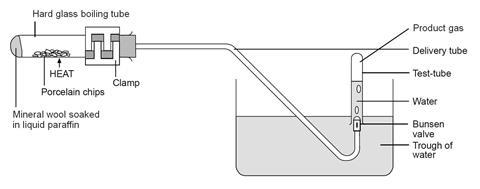
Cracking Hydrocarbons In Liquid Paraffin With A Catalyst Experiment Rsc Education

Oil Refinery Diagram Crude Oil Refining Process Petroleum Engineering Crude Oil Oil Refinery
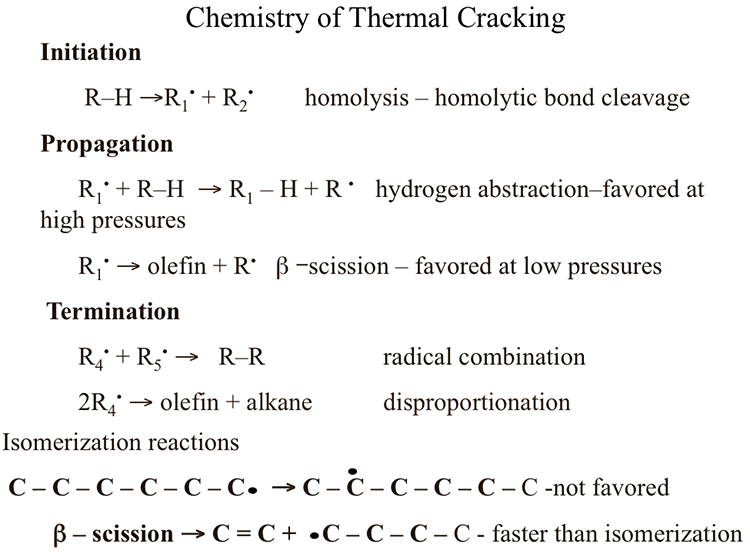
Chemistry Of Thermal Cracking Fsc 432 Petroleum Refining
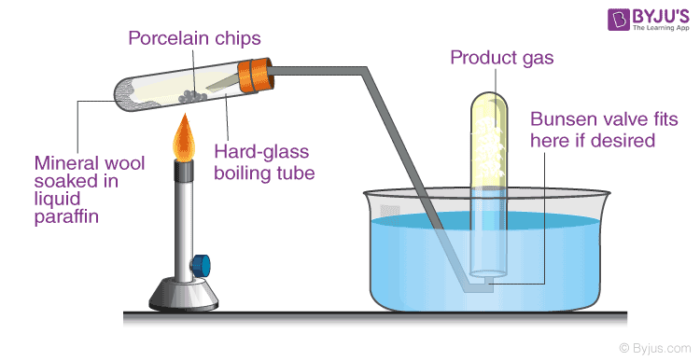
Cracking Meaning Types Of Cracking Organic Chemistry Types

Cracking Hydrocarbons In Liquid Paraffin With A Catalyst Experiment Rsc Education

Posting Komentar untuk "1 Method Of Cracking In Chemistry"