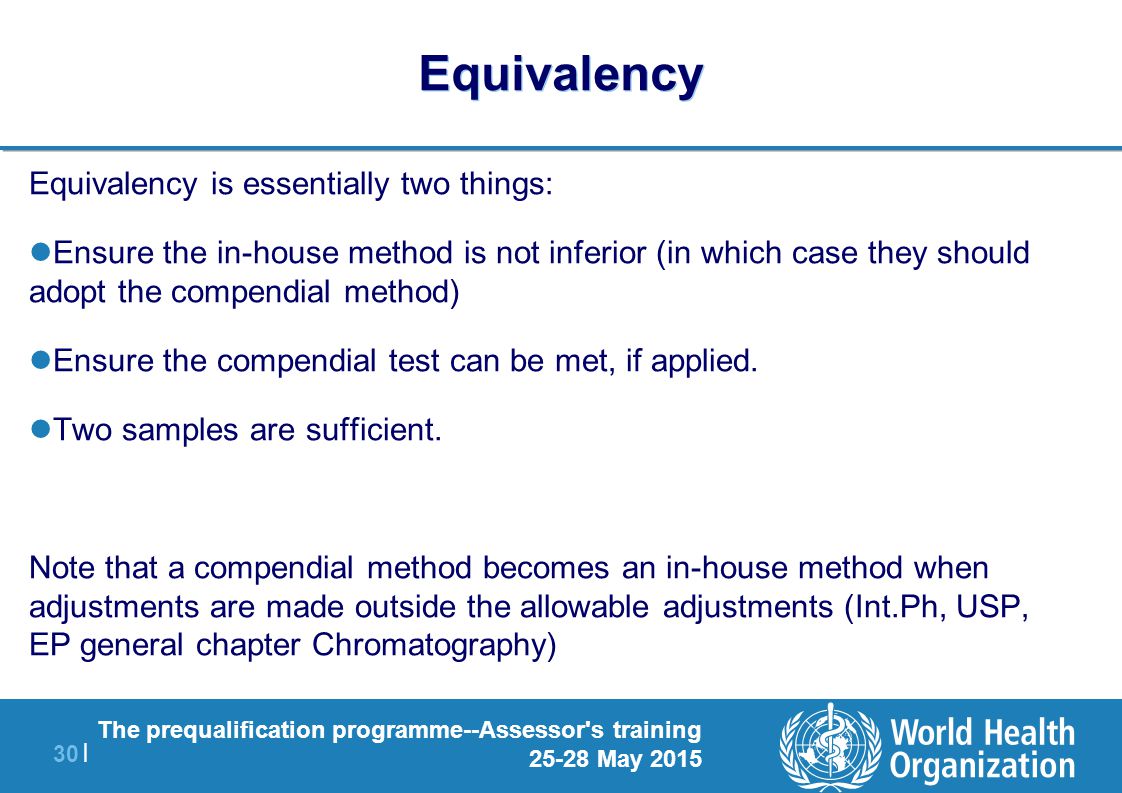
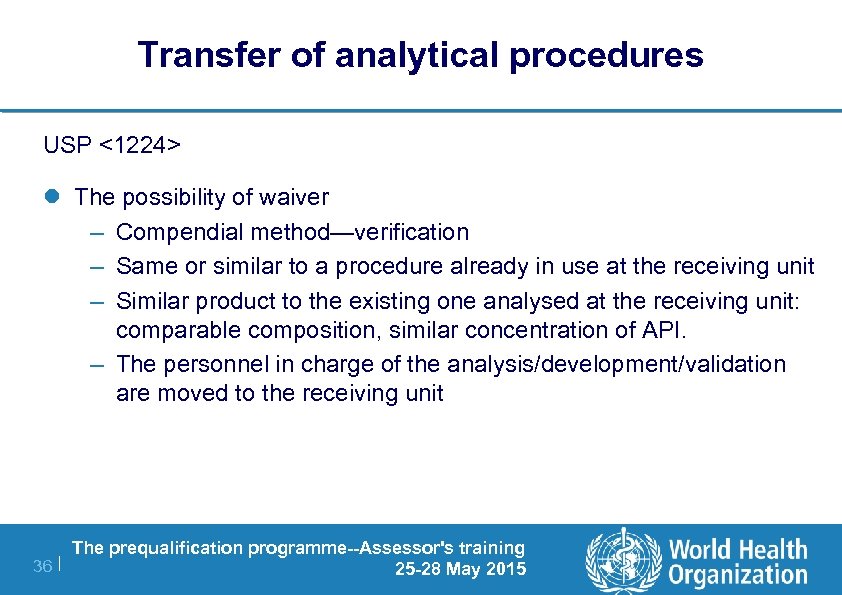
Method Equivalency Usp Chapter
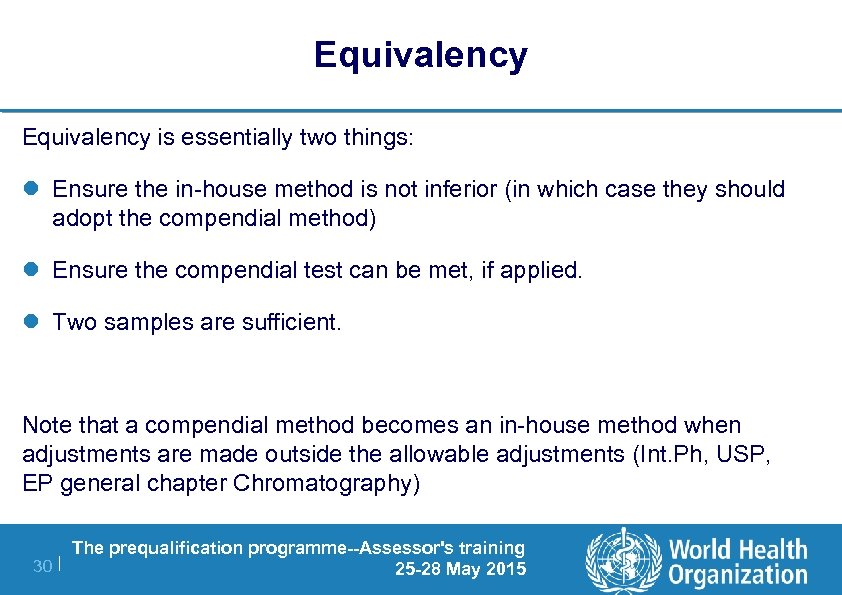
Validation of Compendial Procedures. 5 Compendial methods are verified rather than validated as described in section VI C.
Validation Of Analytical Methods Ppt Video Online Download
Bulk Density and Tapped Density EP.

Method equivalency usp chapter. Newrevised Chapters 220 1200 1210 1220 1225. Allowable Adjustments to United States Pharmacopeia USP Methods As of August 22 2012 Source. -0024 95 lower confidence interval limit for difference.
The residual titration procedure is applicable generally and avoids the difficulties that. We are proposing a Method equivalence study suggestions advice and input is greatly appreciated. What is Method Equivalency.
Alternative method instead of a compendial method equivalency of the alternative method to the compendial method should be demonstrated. USP XXI 1989 served as the foundation for the development of the ICH Q2 Guidance on Validation of Analytical Procedures 1. Strong advocate of this process.
Quotes and Comments from Regulatory Documents USP General Notices 630. Calculate the water equivalency factor F in mg of water per mL of reagent by the formula. WV in which W is the weight in mg of the water contained in the aliquot of standard used.
The purpose of this chapter is to provide guidance for validating methods for use as alternatives to the official compendial microbiological methods. Rev 1 USP. 1225 which was first published in.
For microbial recovery and identification microbiological testing laboratories sometimes use alternative test methods to those described in the general chapters for a variety of reasons such as. Results for the Non-Inferiority Test. USP Sterility KrausePDA 2011 Difference p new method - p EPUSP Estimate for difference.
-008 Statistical Results EPUSP 232 300 077 Candidate 225 300 075 Positives-to-Fail Ratios Method Positives Total Samples. Statistical analysis should be employed for method comparison purposes and USP Chapter Analytical Data Interpretation and Treatment provides consideration for such tools where the focus of equivalency assessment is the methods precision and accuracy variances. For sodium tartrate quickly add 20 1S USP33.
The importance of ICH Q2 and USP chapters USP approach for method validation. Some aspects dissolution drug release which form part of this chapter. Includes measurable parameters and clear criteria Acceptability of a procedure is evaluated by means of.
S6 Rev1 08-May-2007 Link to posting and signoff history. USP Harmonization Status for General Chapters as of 30-Apr-2021 PDG Method Name CP PDG Current Official harmonization Sign-off Status Stage 4 Web Posting Dates G01. And V is the volume in mL of the Reagent used in the titration.
United States Pharmacopeia General Chapter Chromatography USP35-NF30 page 258. The USP method for each of these Methyparaben and Propylparaben is a titration involving the determination of the second. It is done by preparing a study report of comparative data of one representative sample using both methods and prove that in-house method is betterif method.
This Stimuli article discusses approaches for determining equivalent or better procedures. S6 Rev3 06-Nov-2013 Stage. Our Lab hase developed and validated a HPLC assay method for the determination of methylparaben and propylparaben raw material.
Principle See the information given in the section Principle under Method Ia. USP General Chapter 1225 provides specific details about method validation. The concepts and tests discussed in this paper may become one or more USP General Chapters.
Of impurities sensitivity etc. Requirements for Compendial Validation Establishes the types of data that the USP is expecting to see in order to determine the acceptability of a procedure prior to its inclusion in the the Pharmacopeia. The text of this information chapter harmonizes to the extent possible with the Tripartite International Conference on Harmonization ICH documents Validation of Analytical Procedures and the Methodology extension text which are concerned with analytical procedures included as part of registration applications submitted within the EC Japan and the USA.
USP and ICH on method validation and transfer integrated validation verification and validation of analytical procedures for equivalency testing and statistical evaluation. In the residual titration excess Reagent is added to the test specimen sufficient time is allowed for the reaction to reach completion and the unconsumed Reagent is titrated with a standard solution of water in a solvent such as methanol. Basic statistical approaches for evaluating data are described and the treatment of outliers and comparison of analytical methods are discussed in some detail.
Equivalency between two assay methods is quite straight forward by looking at overall RSD between two methods. To ensure compliance it. This chapter provides information regarding acceptable practices for the analysis and consistent interpretation of data obtained from chemical and other analyses.
4 Analytical procedure is interchangeable with a method or test procedure. For Impurity estimation one has to prove that in-house method is equivalent or better than the offical method in terms of resolution no. More recently USP has further led on this topic with the publication of general chapters.
Alternate methods may be used if they provide advantages in terms of accuracy sensitivity precision selectivity or adaptability to automation or computerized data reduction or in other special circumstances.

Analytical Method Equivalency Pharmaceutical Technology
Validation Of Analytical Methods Hua Yin Outline

Pdf Acceptable Equivalent Or Better Approaches For Alternatives To Official Compendial Procedures
Validation Of Analytical Methods Hua Yin Outline
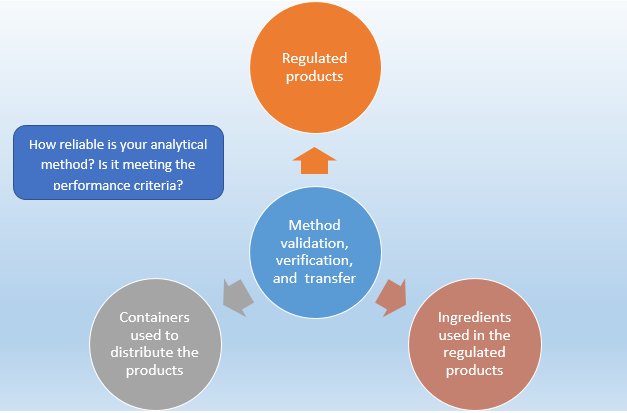
Analytical Method Validation Verification And Transfer Right
Http Www Microbiologynetwork Com Content File Apr 2002 Developing An Information Chapter In The Usp To Demonstrate Equivalency In Microbiological Methods Pdf
.png.aspx?width=300&height=290)
Overview Of Usp 1223 And Ep 5 1 6 Validation Of Alternative Microbiological Methods Particle Measuring Systems

Pdf Acceptable Equivalent Or Better Approaches For Alternatives To Official Compendial Procedures
Are All Methods Equivalent Lachman Consultant Services Inc
Validation Of Analytical Methods Hua Yin Outline
Posting Komentar untuk "Method Equivalency Usp Chapter"